任劲松课题组杨新建同学文章被 Adv. Mater. 接收,文章发表在Adv. Mater. 2012, 24, 2890–2895上。
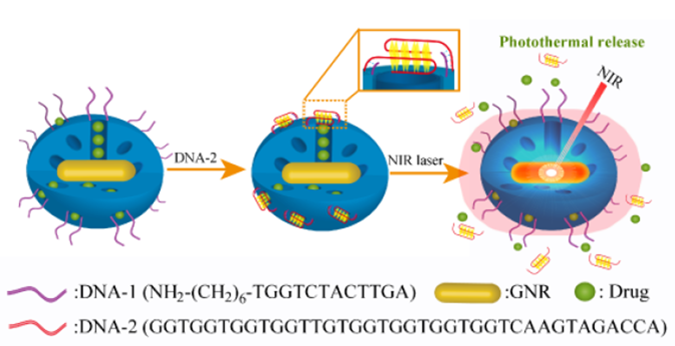
We have demonstrated a novel near-infrared light responsive drug delivery platform based on gold nanorods incorporated within a mesoporous silica framework that was surface-functionalized with aptamer DNA. Upon application to NIR light, the photothermal effect of the AuNRs led to a rapid rise in the local temperature, resulting in the dehybrization of the linkage DNA duplex that anchored the G-quadruplex DNA cap to the surface of the mesoporous silica-based materials, allowing the release of the entrapped guest. In vitro studies have shown the feasibility of using this nanocarrier as a targeted and noninvasive remote controlled drug delivery system in cancer cells with high spatial/temperal resolution. This multifunctional platform could integrate chemotherapy, photothermotherapy and imaging into one system. The good biocompatibility, cancer cell recognition ability and effi cient intracellular drug release provided a basis for in vivo controlled-release biomedical applications and cancer therapy. Importantly, for the fi rst time, we have demonstrated the use of DNA aptamer as both the capping and targeting agent. With aptamer being in principle available for any kind of target, this proof of concept might open the door to a new generation of carrier materials and could also provide a general route to other applications when developing effi cient drug delivery system.