恭喜任劲松课题组陈翠娥文章被Angew. Chem. Int. Ed.接收,文章发表在Angew. Chem. Int. Ed. 2011, 50, 882 –886 上。
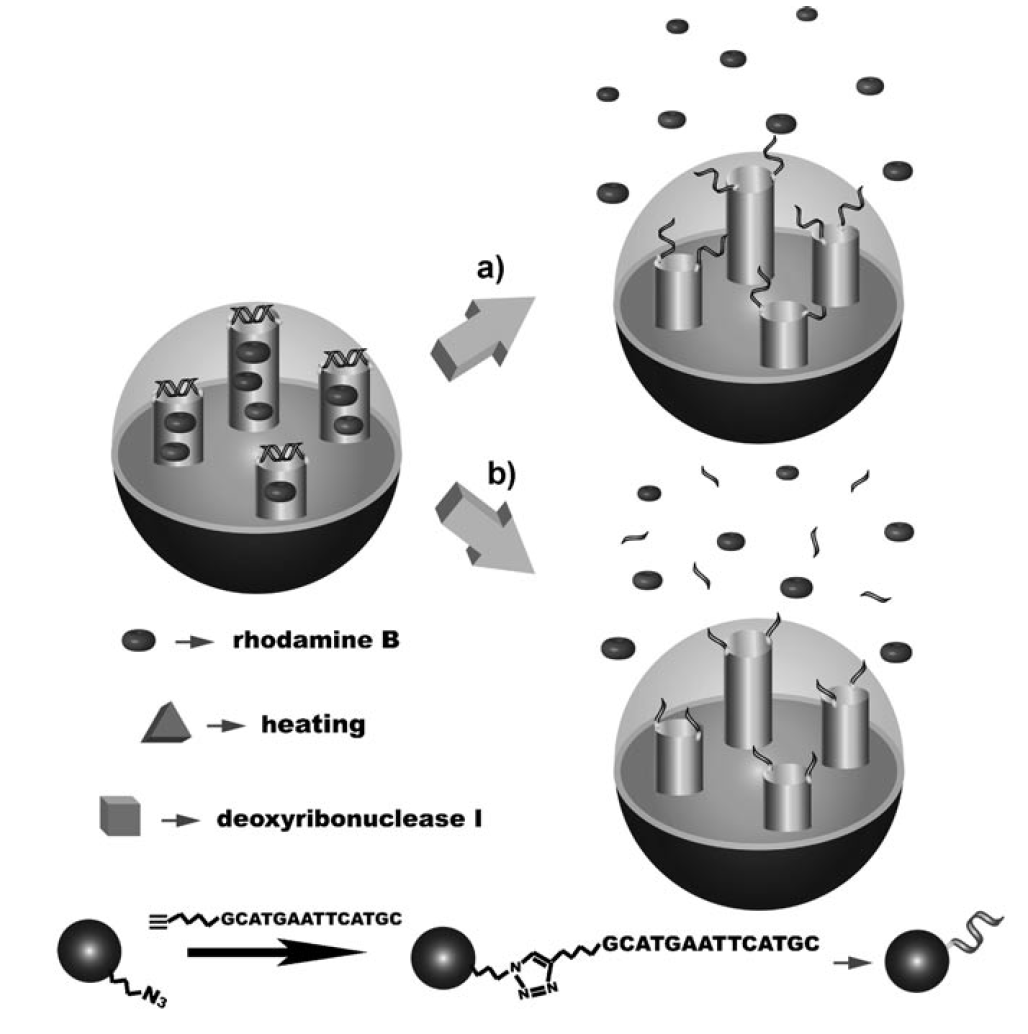
We have designed and synthesized a drug delivery vehicle that is based on DNA-MSPs conjugates and responds to different stimuli. A self-complementary duplex DNAwas attached directly to the outlet of the mesopores by the highly efficient copper(I)-catalyzed azide–alkyne reaction and served as a cap to entrap guest molecules within the mesopores. Cargo release was triggered either by thermal denaturation of the DNA duplex or by the introduction of DNase I to cleave the DNA with high selectivity. Moreover, we have successfully demonstrated that DNA-capped nanoparticles showed a remarkably enhanced efficiency in killing cancer cells, as drug molecules were delivered upon stimulus by endonucleases. The good biocompatibility, cellular uptake properties, and efficient intracellular drug release provide a basis for in vivo controlled-release biomedical applications. This proof of concept might pave the way for a new generation of carrier materials and could also provide a general route for the use of other functional nucleic acids as capping agents in the field of versatile controlled delivery anodevices. In principle, the distinctive sequence-specific properties of DNA could enable the design of nanocontainers that respond to variation of physiological temperature or particular nucleases. Importantly, novel nucleic acids such as aptamers and DNAzymes could be incorporated into this system to construct multifunctional stimulus-responsive devices. Target-directing molecules can also easily be immobilized to the external surface of MSPs to increase the specificity toward cancer cells and minimize toxicity to the surrounding normal tissues.